
Recently, researchers worked to the feasibility of engineering phytases with resistance to boiling using rational design strategies. The findings are published in Journal of Biological Chemistry.
Phytase has been authorized as a feed additive for over 2 decades and is widely recognized in the market as the predominant feed enzyme, constituting 60% of the entire industry. By hydrolyzing phytate—a compound that inhibits nutrient absorption found in animal feed—into inorganic phosphoric acid, it effectively reduces phosphorus excretion while enhancing mineral and protein digestion and absorption. As a result, this enzymatic process significantly improves animal production performance. However, the issue of heat resistance has emerged as a critical bottleneck hindering the industrial application of phytase due to the technical requirement of instantaneous high temperatures during feed pelletizing processes.
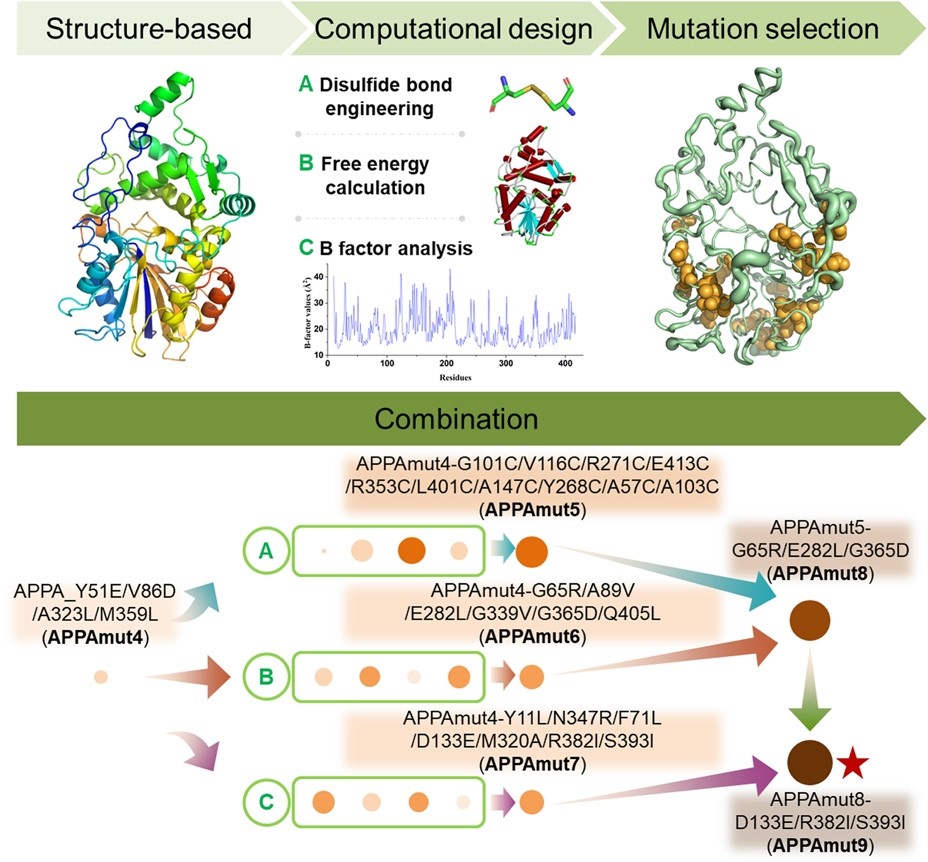
Figure 1 Schematic representation of computational design based on the structural basis of APPAmut4.
Rational design strategies were employed involving the introduction of disulfide bonds, free energy calculation, and B-factor analysis based on the crystal structure of phytase APPAmut4 (1.90 Å), a variant with enhanced expression levels derived from Yersinia intermedia, to improve its thermostability. Through iterative combination mutations, a variant APPAmut9 was obtained with enhanced thermostability while maintaining its enzyme activity. APPAmut9 exhibited a T50 value of 96 °C, representing a substantial increase of 40.9 °C compared to APPAmut4. Remarkably, this variant retains 70% of its activity even after exposure to the extreme temperature of 100 °C for 5 min, showcasing an exceptional level of thermal resilience rarely observed in the realm of enzyme engineering research. This study successfully achieved thermostability of a phytase with resistance up to 100 °C, making it a promising candidate for industrial applications requiring high-temperature enzyme activity.
The online version available at:
https://www.jbc.org/article/S0021-9258(24)02494-3/fulltext

