A recent study conducted by the Livestock Nutrition and Regulation Research Team has elucidated the mechanism through which dietary xylooligosaccharides (XOS), mediated by Lactobacillus reuteri (L. reuteri), enhance jejunal cell survival in piglets. The findings offer a novel strategy for the nutritional regulation of intestinal health in weaned piglets and have been published in iMeta.
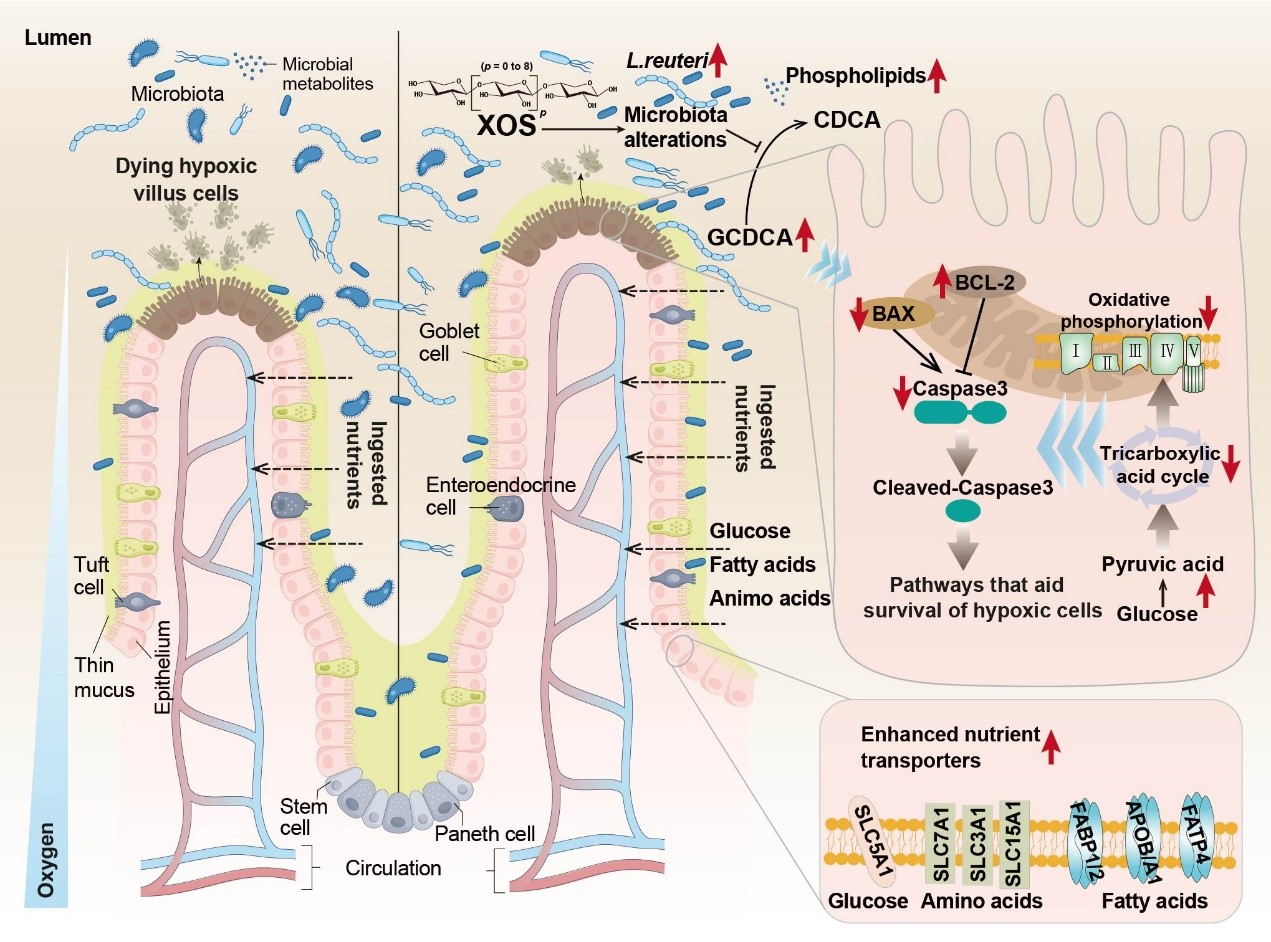
A balanced gut microbiota is essential for the growth and health of piglets. Functional oligosaccharides, such as XOS, are known to modulate gut microecology by promoting the growth of beneficial bacteria. While these oligosaccharides have traditionally been regarded as "passers-by" in the small intestine—primarily functioning through fermentation in the hindgut—this study demonstrates that XOS actively interact with the small intestinal microbiota to improve jejunal cell survival. This discovery expands the known functional scope of dietary oligosaccharides and offers new insights into intestinal health regulation.
The results showed that dietary XOS supplementation improved the growth performance of weaned piglets by increasing jejunal villus height and enhancing nutrient transporter expression. Further mechanistic studies revealed that XOS act not by stimulating cell proliferation, but by inhibiting apoptosis in jejunal epithelial cells, thereby boosting epithelial cell survival. This anti-apoptotic effect was attributed to reduced cellular oxygen dependence. Importantly, L. reuteri—enriched by XOS in the small intestine—was identified as a key mediator of this process. In subsequent validation experiments, L. reuteri was shown to alleviate E. coli-induced apoptosis and mitigate E. coli-associated weight loss in piglets. Integrated multi-omics analysis and targeted metabolomics validation identified glycochenodeoxycholic acid (GCDCA), a bile acid in jejunal chyme, as positively responsive to both XOS supplementation and L. reuteri enrichment. Additional in vitro experiments confirmed that low-dose GCDCA suppressed hypoxia-induced apoptosis in porcine jejunal epithelial cells (IPEC-J2). These results collectively reveal a new mechanism: XOS, by enriching L. reuteri in the jejunum, remodel the energy metabolism of intestinal cells and inhibit epithelial cell apoptosis under hypoxic conditions. The study provides a scientific basis for the precise nutritional management of intestinal health in animals.
The co-first authors of the study are Ph.D. candidates Deng Fuli and Yin Chang, and postdoctoral researcher Luo Chengzeng. The co-corresponding authors are Researcher Zhang Hongfu, Researcher Chen Liang, and Associate Researcher Tang Shanlong, all affiliated with the Institute of Animal Science, Chinese Academy of Agricultural Sciences. The research was supported by the National Natural Science Foundation of China and the Agricultural Science and Technology Innovation Program of China.
Link to the original article: http://doi.org/10.1002/imt2.70080