IAS Revealed the Molecular and Cellular Mechanism of Nonalcoholic Steatohepatitis in Bama Minipigs Induced by a Long-Term High-Fat, High-Sucrose Diet
Source: Author:Yang Shulin Date:2015-03-19
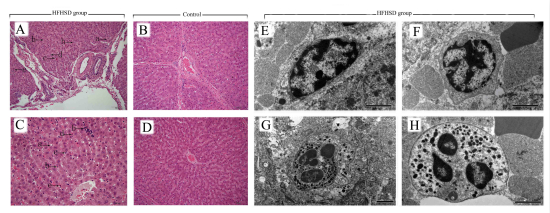
Obesity, metabolic syndrome and the associated chronic inflammation are among the most prevalent diseases that impose enormous health and economic burdens on governments around the world and on the global economy. Inflammation is believed to be the driving force behind the development of NASH and also acts as a critical predictor of the histological progression to fibrosis and cirrhosis; thus, inflammation represents a potentially major therapeutic target in NASH. Several species have been used as models for obesity and NASH, and rodents have been established as the primary model for these diseases. However, there are metabolic and physiological differences between humans and rodents that have undoubtedly slowed progress and complicated this research. Pigs are rapidly emerging as a biomedical model for obesity and energy metabolism in humans because of their similar metabolic features, cardiovascular systems, and proportional organ sizes. We established the metabolic syndrome with Bama minipigs after being fed a high-fat, high-sucrose diet (HFHSD) for 23 months; the early signs of NASH, steatosis, oxidation stress, iron overload, lipid peroxidation and cellular damage all can be observed in the liver.Compared with the control group, there was a significant increase in the number of inflammatory cells, including lymphocytes, eosinophils, neutrophils, and Kupffer cells, in hepatic lobules in the HFHSD pigs. Through the analysis of the RNA-Seq data pathways, the process of inflammation involved the inflammatory signal transduction-related toll-like receptor, MAPK, and PPAR signaling pathways; the cytokine-related chemokine signaling, cytokine-cytokine receptor interaction, and IL2, IL4, IL6, and IL12 signaling pathways; the leukocyte receptor signaling-related T cell, B cell, and natural killer cell signaling pathways; inflammatory cell migration and invasion related pathways; and other pathways. Moreover, we identified several differentially expressed inflammation-related genes between the two groups, including FOS, JUN, TLR7, MYC, PIK3CD, VAV3, IL2RB and IL4R, that could be potential targets for further investigation.
The choice of animal disease model plays an important role in the study of the hepatic inflammation molecular mechanisms of NASH. From this study, the minipigs exhibited obesity, hyperinsulinemia, dyslipidemia, and inflammation accompanied by significantly increased levels of inflammatory cells. Differences in genes and pathways clearly indicated progressive signal transduction, directed toward the invasion of inflammatory cells, and toward leukocyte transendothelial migration. The results of this study indicated that the minipigs should be of good models for the future study and the development of new drugs of NASH.
This research was completed by the researcher at Research team of pig genetic engineering and germplasm innovation, Institute of Animal Sciences, mainly supported by the National Natural Science Foundation of China (31372276) and Agricultural Science and Technology Innovation Program (ASTIP-IAS05) etc. The research result was published in PLoS One. 2014 Nov 21;9(11):e113724. The target selection team at Global Medical Discovery has identified this publication as a Key Scientific Article contributing to excellence in biomedical research and featured it in the Global Medical Discovery Series on March 11, 2015.
https://globalmedicaldiscovery.com/key-scientific-articles/transcriptome-analysis-on-the-inflammatory-cell-infiltration-of-nonalcoholic-steatohepatitis-in-bama-minipigs-induced-by-a-long-term-high-fat-high-sucrose-diet/
By Yang Shulin
Yangshulin@caas.cn
Yangshulin@caas.cn